

Across the chemical and energy sectors, efficiency and reliability are paramount. Few catalysts deliver this as consistently as copper-zinc-aluminum (Cu-Zn-Al) formulations. These versatile workhorses drive critical reactions fundamental to producing everyday materials and clean energy. Understanding their role is key for anyone involved in sourcing catalysts for methanol plants, hydrogen generation, or gas purification.
The Cu-Zn-Al Advantage: Synergy in Action
At the heart of these catalysts lies a powerful synergy:
Copper (Cu): The primary active site, facilitating key reactions like hydrogenation and carbon oxide conversion.
Zinc Oxide (ZnO): Stabilizes the copper particles, prevents sintering (deactivation by particle growth), and can enhance activity.
Alumina (Al₂O₃): Provides crucial structural support (high surface area), improves mechanical strength, and enhances thermal stability.
This combination results in catalysts highly optimized for moderate-temperature operations (typically 200–300°C), offering significant advantages over older, less efficient, or more expensive alternatives.
Core Applications Driving Industry
Methanol Synthesis: The Cornerstone Application
The vast majority of the world's methanol – a vital feedstock for plastics, fuels (MTBE, DME), formaldehyde, and more – is produced using Cu-Zn-Al catalysts. They efficiently convert synthesis gas (CO + 2H₂ → CH₃OH) at lower pressures (50-100 bar) than older technologies, offering superior activity, selectivity, and cost-effectiveness.
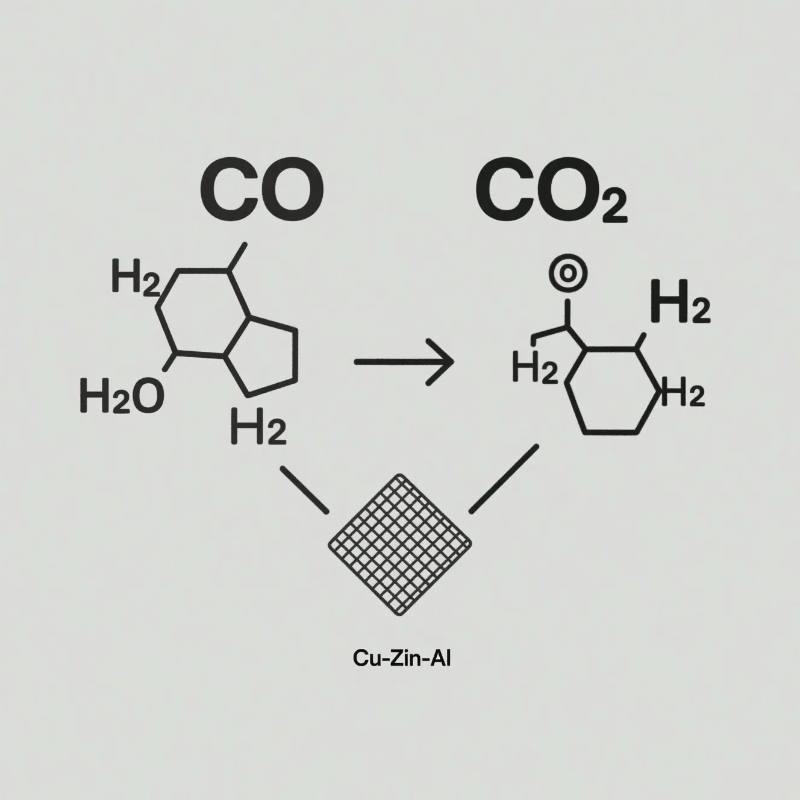
Water-Gas Shift (WGS) Reaction: Purifying & Producing Hydrogen
Essential in hydrogen plants, ammonia synthesis, and refinery operations, Cu-Zn-Al catalysts are the workhorse for the Low-Temperature Shift (LTS) reaction:
CO + H₂O → CO₂ + H₂
They excel at converting toxic carbon monoxide into additional hydrogen and easily removable CO₂, crucial for hydrogen purity and yield, operating effectively around 200-250°C.
Methanol-to-Hydrogen (MTH) Reforming: On-Demand Hydrogen
For fuel cells, portable power, and smaller-scale applications, methanol steam reforming using Cu-Zn-Al catalysts provides a practical hydrogen source:
CH₃OH + H₂O → 3H₂ + CO₂
Their high activity, good hydrogen selectivity, and stability make them ideal for compact reformers requiring quick start-up and reliable operation.
Selective Hydrogenation:
Used in fine chemicals and petrochemical processing, Cu-Zn-Al catalysts selectively hydrogenate compounds like aldehydes (e.g., converting furfural to furfuryl alcohol) or remove trace impurities like acetylene from ethylene streams without over-hydrogenating the main product.
Key Benefits Driving Adoption
High Activity at Lower Temperatures: Reduces energy consumption and operational costs.
Excellent Selectivity: Minimizes unwanted byproducts, improving yield and reducing downstream separation costs.
Proven Cost-Effectiveness: Offers a high-performance alternative to precious metal catalysts.
Robust Formulations: Can be tailored for specific process requirements (e.g., different syngas compositions, space velocities).
Long Operational History: Well-understood performance and regeneration protocols.
Critical Considerations for Performance
Sulfur Sensitivity: Cu-Zn-Al catalysts are highly susceptible to poisoning by sulfur compounds. Effective feedstock purification (desulfurization) is mandatory.
Oxidation Risk: Copper is pyrophoric in its active reduced state. Care must be taken during handling, loading, unloading, and especially during start-up/shutdown to avoid exposure to air.
Proper Activation: The catalyst precursor requires careful in-situ reduction with hydrogen to activate the copper sites.
Securing Consistent, High-Performance Catalysts
The performance of processes like methanol synthesis or hydrogen purification hinges directly on the quality, consistency, and reliability of the Cu-Zn-Al catalyst supply. Precise control over composition, particle characteristics, and activation protocols is non-negotiable for achieving design capacity, efficiency, and longevity.
We specialize in connecting industry with rigorously sourced Cu-Zn-Al catalysts for these demanding applications. Ensuring access to materials that meet exact specifications, backed by reliable supply chains and clear performance data, is fundamental to maintaining optimal plant operations and productivity. Consistent catalyst quality is not just a purchase; it's an investment in process stability and output.