

Hydrogen is often called the fuel of the future, but much of it is produced today through a process called steam reforming. This method converts hydrocarbons—primarily methane from natural gas—into hydrogen and carbon oxides. At the core of this chemical transformation lies a critical component: the catalyst. In particular, nickel-based catalysts serve as the industrial standard, enabling efficient, large-scale hydrogen production.
Why Steam Reforming?
Steam reforming is the most established and cost-effective route for commercial hydrogen production. It supplies hydrogen for refining petroleum, making fertilizers, and increasingly, for clean energy applications. The process involves reacting hydrocarbon fuels with steam at high temperatures. The most common feed is methane (the main component of natural gas) in Methane Steam Reforming (MSR), but the technology can also be adapted for other hydrocarbons.
The Catalyst’s Crucial Role
A catalyst speeds up a chemical reaction without being consumed. In steam reforming, the catalyst must perform under demanding conditions—high temperatures (700–1000°C) and in the presence of steam—while maintaining activity and stability for years.
The Preferred Choice: Nickel on a Stable Support
Nickel is the active metal of choice for most industrial steam reforming catalysts because it offers an excellent balance of high activity and relatively low cost compared to precious metals like platinum or ruthenium.
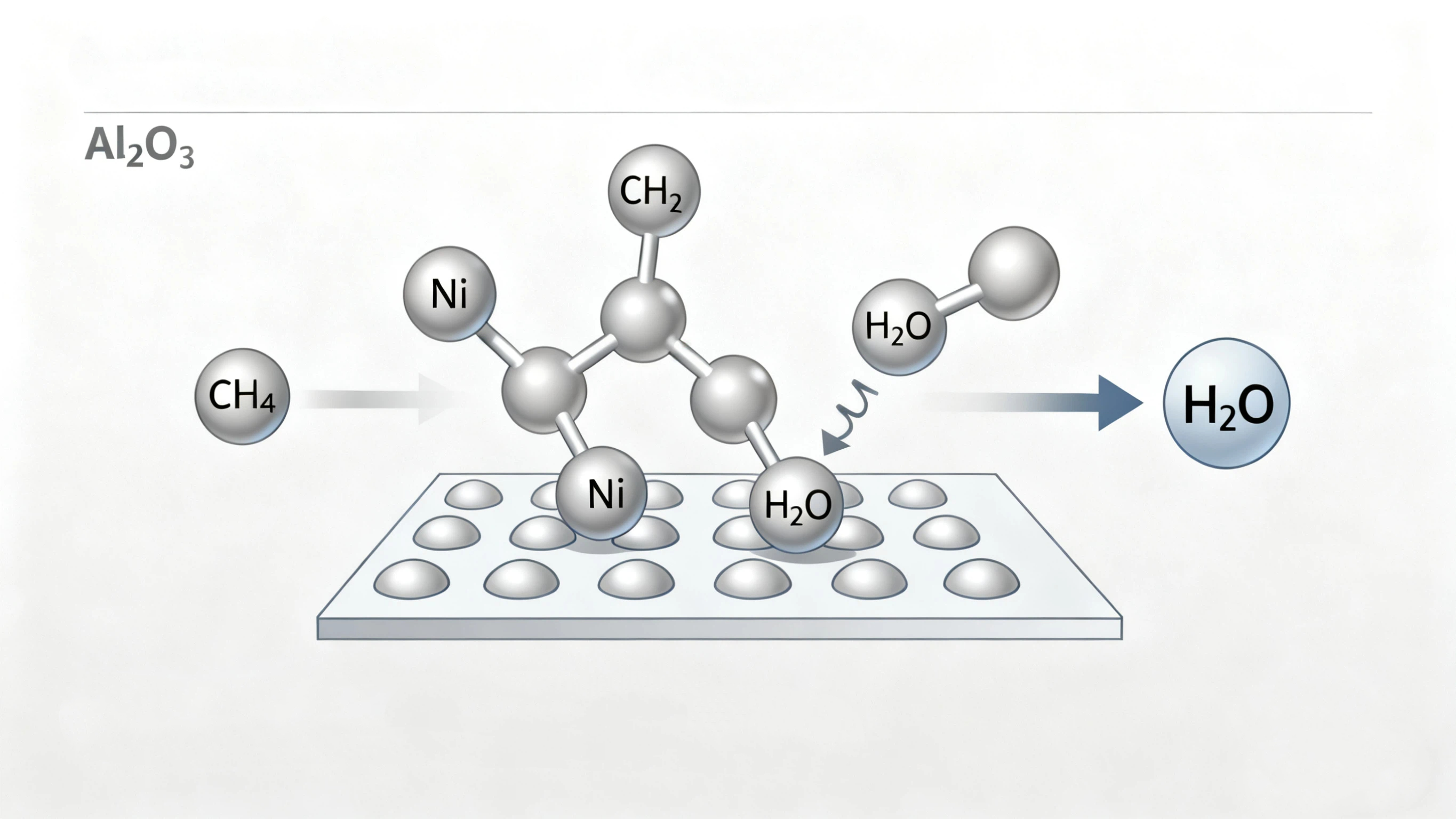
The Chemical Heart: Methane Steam Reforming (MSR)
The
primary reaction, simplified, is:
CH₄ + H₂O → CO + 3H₂
This is followed by the water-gas shift reaction to produce more hydrogen from
the carbon monoxide.
The nickel catalyst’s job is to ensure this reaction proceeds rapidly and completely. A key engineering challenge is suppressing side reactions that form solid carbon deposits ("coking"), which can deactivate the catalyst. Modern nickel catalysts are often promoted with small amounts of other elements (e.g., magnesium, potassium) to resist coking and enhance longevity.
Beyond Methane: Hydrocarbon Steam Reforming
The same catalytic principle applies to reforming heavier hydrocarbons, such as liquefied petroleum gas (LPG) or naphtha. The chemistry is more complex due to larger molecules and a higher risk of carbon formation. Here, the catalyst formulation—especially the choice of support and promoters—becomes even more critical to ensure clean, efficient operation without deactivation.
Why This Matters for the Energy Transition
While the ultimate goal is to produce "green hydrogen" from renewable electricity, steam reforming of natural gas with carbon capture (a pathway known as "blue hydrogen") is seen as a crucial transitional technology. It can scale up clean hydrogen supply using existing infrastructure. Improvements in catalyst efficiency, durability, and resistance to poisoning directly lower the cost and environmental footprint of this hydrogen.
In Summary
Nickel-based steam reforming catalysts are a cornerstone of modern industrial chemistry. Their robust design, combining an active nickel phase with a stable, engineered support, allows for the reliable and economical production of hydrogen from hydrocarbon resources. As the world navigates its energy transition, these catalysts will continue to evolve, playing a vital role in building the hydrogen economy.